Which aspects of the cell factory systems are mainly used in biopharmaceuticals
In the field of biopharmaceuticals, cell culture consumables are essential tools. Among them, the cell factory systems has become a commonly used consumable in biopharmaceuticals due to its small footprint, reduced pollution. It is mainly used in small test, pilot test, mass production and other links.
Small test, pilot test, and scale-up are three very important links in biopharmaceuticals. The small test is scaled up according to the laboratory effect. The pilot test is the test before the product is officially put into production, and it is a small-scale test of the product before mass production. Master the basic parameters of the process in the small test stage, write the pilot test plan on this basis, and carry out the pilot scale test. After the pilot test is over, the existing process of the small test will be improved and improved according to the phenomenon and results of the process amplification. In addition, the operating procedures for product production are formed. After the procedures have been verified by more than three consecutive batches of processes, they can be used as the process basis for large-scale production. The processes of each stage are inherited in one continuous line.
The specifications of the cell factory systems used in different stages will be different. In the small-scale test stage, 1-layer and 2-layer small specifications are mainly used, while the pilot test will use 5-layer and 10-layer specifications, and the mass production stage is mainly based on 40-layer cell factory systems.
In general, cell factory systems of different specifications are used in different stages of biopharmaceuticals. It is worth mentioning that in the later stage of large-scale production, it can be matched with the corresponding pipeline system to realize the airtight transfer of liquid and reduce the risk of external source pollution during operation.
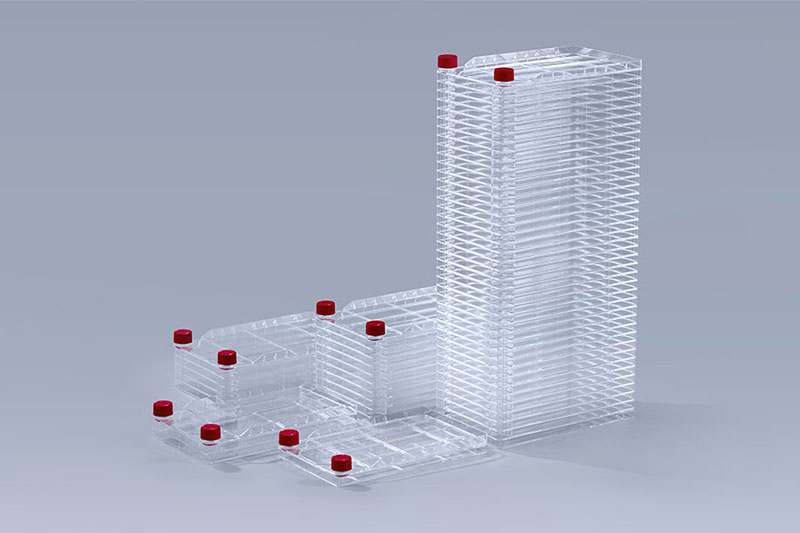 Cell Factory Systems
Cell Factory Systems
评论
发表评论