Passage operation of cells in cell factory systems
Cell passage is a common operation in cell culture, which refers to the transfer of cells from one culture vessel to a new culture vessel to promote continued proliferation and growth of cells. The following are the general steps of the cell passaging operation in the cell factory systems:
1. Prepare the cell culture: Remove the cell culture and check the status and density of the cells. Ensure cell cultures are growing optimally and assess cell numbers and health.
2. Pretreatment of the culture container: select the cell factory systems with appropriate specifications, and add an appropriate amount of culture medium or culture solution according to the number of cells and the expected growth time.
3. Rinse the cells: Rinse the cells in the cell culture with the culture medium to remove the residue and waste liquid in the cell culture. Rinse is usually performed with a sterile buffer such as PBS (phosphate buffered saline) or sterile cell culture medium.
4. Cell dissociation: add appropriate cell dissociation enzyme, enzyme amine or mild buffer to dissociate cells so that they can be dispersed into single cells. Times and conditions should be optimized for specific cell types and dissociation reagents.
5. Count cells: Use a cell counter or reciprocator to count the dissociated cells to determine the concentration and number of cells. Calculate and adjust the passage ratio of the cells as needed.
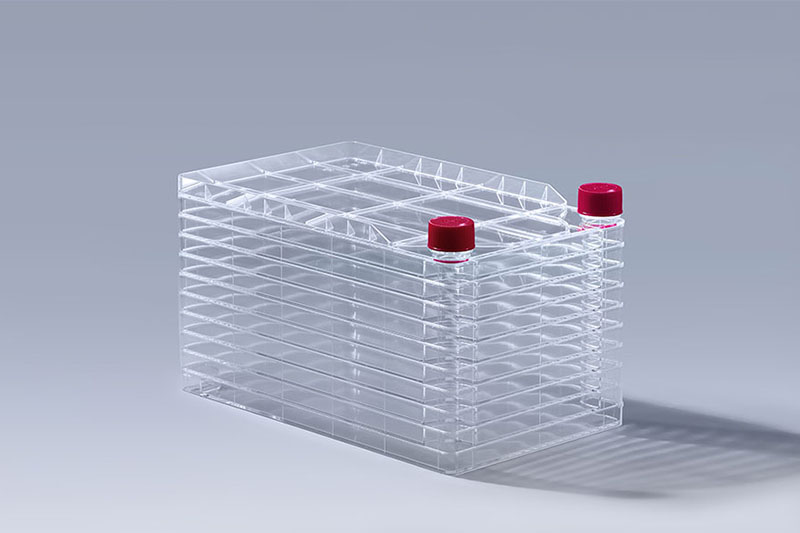 10 Layers Cell Factory Systems
10 Layers Cell Factory Systems
6. Inoculate a new culture container: Add an appropriate amount of cells into the pretreated new cell factory systems to continue culturing.
The above are the operation steps of cell subculture in the cell factory systems. We must strictly follow the aseptic principle during operation, because the cells are easily contaminated due to improper operation. Of course, you can also use the dedicated piping system of the cell factory systems to reduce the risk of cell contamination.
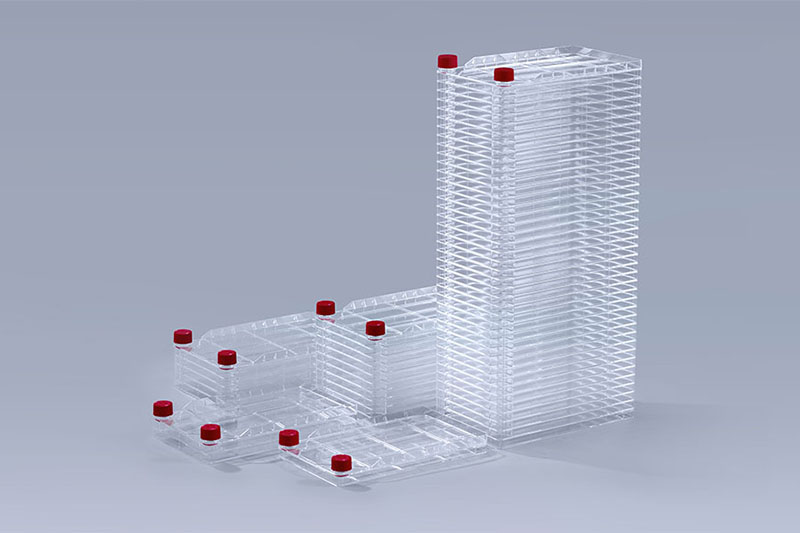 Cell Factory Systems
Cell Factory Systems
评论
发表评论